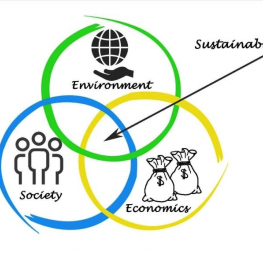
What is classified as sustainable or recyclable packaging?
The Sustainable Packaging Coalition (SPC) believes that industries can use their influence to improve sustainability in packaging. The SPC envisages:
“a world where all packaging is sourced responsibly, designed to be effective and safe throughout its lifecycle, meets market criteria for performance and cost, is made entirely using renewable energy, and once used, is recycled efficiently to provide a valuable resource for subsequent generations (Definition of Sustainable Packaging).”

They do this by researching and using scientific methodologies to advance and support a positive environmental vision for packaging while supporting cutting-edge, useful packaging products and systems that promote economic and environmental health. To classify a package as sustainable, they have to meet the following criteria (in no specific order or importance):
- Is safe for the health of individuals and communities and beneficial throughout its life cycle.
- Meets market standards for performance and cost.
- Is obtained, produced, distributed, and recycled using renewable energy.
- Maximizes the utilization of recycled/renewable resources.
- Is made with best practices and eco-friendly technologies.
- The materials used to make it are robust throughout its life cycle.
- Is physically built to maximize materials and energy.
- Is recovered effectively and used in biological or industrial closed loop cycles.
The SPC understands that this criterion will likely only sometimes be met. However, they would use this to characterize what they are striving for. For further interest in the criterion and their significance to packaging, more can be found in the “Definition of Sustainable Packaging” by the Sustainable Packaging Coalition.

What is important about packaging and what are some examples of regulations placed on it?
Reusable medical devices (RMD) are packaged to maintain sterility until usage. To accomplish this goal, the packaging system must have a microbial barrier, protect the RMD and microbial barrier's integrity from the distribution handling, be compatible with the specified sterilization process, and allow for aseptic presentation of the RMD. Aseptic presentation refers to a sterile product's introduction and transfer using noncontaminated procedures and conditions ("Packaging"). Collaborating with a surgical or medical user, a packaging process is developed based on the sterilization department's RMD characteristics, organizational structure, and experience. The RMD's configuration and weight influence the size and resistance of the packaging system. Additional shielding layers are added to the packaging to increase its physical resistance. Protective coatings can also prevent environmental dust from building up on the package's surfaces. The preservation of an RMD and its packaging integrity is aided by the methods and tools used in transportation and storage, such as cabinets and shelves. The packaging components must be compatible with the specified sterilization process. Some items that need to be considered are the ability to preserve the microbial barrier's properties, the package's physical resistance to pressure variation and high temperatures, the lack of toxic by-products, and the release of particles. The international standard (ISO) that defines a package and the aseptic information is ISO 11607 ("Packaging"). This can be further looked into for more details on the requirements that need to be met for each package categorization. Multiple packaging methods contain sterile barriers, such as single-use wraps, pouches, reusable containers, and textiles. Single-use wraps are made of cellulose, synthetic, or mixed cellulose/synthetic sheets that are woven or nonwoven and are primarily used for baskets and instrument sets. They involve multiple folding methods secured by adhesive tapes, which also serve as sterilization indicators. Single-use pouches contain two sheets pre-sealed on three sides of the pouch, and the pouch's closure is done by heat sealing. They are offered in multiple dimensions and strengths and utilized for lightweight objects. The reusable containers are made of aluminum, stainless steel, or polymers. Container cleaning and disinfection must follow instructions for use (IFU) guidelines, which require organization, resources, and tools. Disposable products are thrown away, and reusable accessories are removed before reprocessing. Various variables are inspected thoroughly on containers, such as cleanliness, filtering, impact, and defectiveness, to determine whether to cleanse containers or send them off for reparations. Several manufacturers advise single-use filters. Reusable filters are examined, and any found to be flawed are discarded. The filters are discarded when the manufacturer's specified maximum number of reprocessing cycles is reached. Along with reusable containers, there are also reusable textiles made of cotton or polyester. The sterilization barrier performance of the traditional cotton wraps is inadequate and deteriorates with repeated usage ("Packaging"). To avoid that challenge, polyester wraps are used because of their better performance. To have efficient sterilization and preservation of purity, the packaging process must be compatible with the RMD. Risk analysis and validation checks could be conducted to determine whether the packaging process is effective.
What are criteria for a package to be considered sterile?
This article has listed multiple steps to be taken when developing packaging for a sterilized medical product. A few of the steps are as follows: 1) Listen to consumers, 2) Review regulations, 3) Determine real-world conditions, 4) Define product package requirements, etc. (Larsen). This exemplifies the idea that sustainable packaging must be implemented more quickly. When bringing a new product to the medical device, there are testing requirements, documentation that needs to be submitted, and then processes to be adjusted.
Packaging sterility has criteria that need to be met when dealing with reusable medical devices as well. There is a standard operating procedure set in place, and some elements that the packaging has to meet are the same as stated before, such as being a barrier to micro-organisms, permitting air removal and steam penetration, and having enough strength to withstand distribution logistics. More items need to be evaluated, such as not mixing stainless steel items with linen within the same package and not using staples, pins, or elastics to enclose a box. There are more steps to be followed in this procedure, and it is also important to note that this is specific to Alberta Health Services (“Reusable Critical Med Devices”).

Sustainability in medical device/healthcare packaging:
A packaging engineer, Jennifer Griffin, from Medtronic, conveys some steps one of the most known medical device companies took to become more sustainable and eco-friendlier (Sparrow). In her presentation, she spoke about revamping some secondary packaging items. The new packaging designs reduced the items' size and weight because they moved from a dual to a single sterile barrier. The modifications of the packages resulted in a reduction percentage of 45 to 85 (Sparrow). This change led to benefits for the company, such as material reduction, sterilization, shipping cost, and storage. The engineer also made a connection between packaging needs and customer wants. She spoke about packaging redesigns and receiving consumer feedback on what they preferred and would choose, which they chose the smaller package. She also mentioned how a hospital decided to use their (Medtronic) instruments because of the size of the package. It was also explained that design improvements made on the device itself can also lead to a change in the packaging component (Sparrow). There was a case that involved a redesign of a medical device, which led to a reduction in packaging size because it had to be narrower. Griffin noted that there is some hindrance to becoming more sustainable due to practitioner and patient safety and liability issues.

Sustainable packaging is something that every industry should be striving for; however, it is noticed that the healthcare industry is hesitant to participate in this innovative method. The hesitance to join is because some packaging material does come in contact with hazardous substances. However, there is an estimation that "hospitals produce thirty pounds of waste per bed every day" ("Sustainable Healthcare Packaging: Why Recyclability Is the Key"). Most of this waste is nonhazardous and could be recycled if appropriate practices were set in place. More recently, mono-material solutions — one type of recyclable plastic with existing capabilities — are suitable for focusing on the product's protection and the patient's safety. For example, medical forming films use multi-layer nylon/PE or ionomer/PE. They can be replaced with a polyethylene-based single material with the same characteristics to endure the sterilization processes needed for medical devices and be recyclable ("Sustainable Healthcare Packaging: Why Recyclability Is the Key"). Another example would be blister packaging, where a PVC/foil combination is used and cannot be recycled. Many companies are using a mono-material system as its replacement. Manufacturers are producing packaging that maintains moisture barrier properties that the PVC/foil would have, as well as reducing the carbon footprint and consumption of non-renewable energy by 76% and 77%, respectively ("Sustainable Healthcare Packaging: Why Recyclability Is the Key"). Using high-density polyethylene (HDPE) as a mono-material instead of other non-sustainable materials has more benefits and would aid in meeting sustainability goals. From a manufacturing view, HDPE does not require tooling changes, supply chains can be simplified, and quality can be sustained.

Introduction of recycled material into Tyvek:
DuPont had a challenge with Tyvek, a nonwoven polyethylene textile product, because of its environmental impact. Tyvek is used in numerous applications, from medical packaging to construction of buildings. However, DuPont committed in the 1990s to strive for zero waste and emissions to the reuse and recyclability their materials to conserve resources (Sharfman). One of the problems the company faced was their pride in the quality of their products. After many years of manufacturing Tyvek, they finally reached their quality goal. This wasn't easy to deal with when the company tried an innovative method of implementing recycled material into the product line. The production team rejected the idea because of how smoothly everything was running. After overcoming that obstacle, the next was the inadequate purity level in the recycled material. DuPont spent a lot of time working with companies that could yield HDPE that contained the required purity and quality (Sharfman). Finally, the production phase was finalized using extruders, better filters, and washing systems. After this, more problems arose, with the printers and contamination of sand, deterring the implementation of more sustainable practices. As seen in this case, multiple environmental innovation concepts, including the roles that institutional structures and the business market have, signify the importance of working with the entire supply chain and the need for an interdisciplinary team to address the complex nature of environmental problems. Although the introduction of PCR Tyvek has been successful in the envelope market, it has been hindered or limited in other important Tyvek industries, such as house wrap, medical packaging, and protective gear (Sharfman). Even though there were better methods for the company than introducing recyclable material, they showed interest in exploring the lifecycle of Tyvek and what environmental advantages it could bring to the industry.

Challenges and strategies of sustainable packaging:
A few challenges of sustainable packaging when trying to implement them are: they can be more expensive than processes currently being used, because of the previous reason consumers may not want to pay more, sustainable packaging is sometimes not compatible with some products, it can affect supply chain and logistics, and lastly it has different regulations and standards. A way to overcome the expensive costs is to use life cycle analysis (LCA). This tool assesses the environmental impacts of products and systems through their lifecycle, from beginning to end (Richards). It can determine flexible packaging, such as identifying places in the lifecycle that can be more sustainable, comparing alternatives, and increasing efficiency. To persuade consumers to pay the extra charges for items, companies could educate the consumer population on the multiple benefits of sustainable packaging. Industries must stay updated on new regulations and standards on sustainable packaging, such as extended producer responsibility (EPR) (Richards). This policy holds manufacturers responsible for environmental impacts caused by their products throughout their whole lifecycle.

Although plastics are helpful, the problem is that all plastic used in the medical industry must be brand-new, virgin material to guarantee that the product packaging is hygienic and sterile. PETG medical packaging is recyclable in some circumstances; however, it cannot be recycled if it becomes contaminated and must be disposed of in a biohazard container (Haub). Finding strategies to make medical plastic a sustainable alternative is more complex than in other industries because employing recycled plastic is sometimes not an option, and only a few materials can be recycled. Companies should focus more on sustainable operations instead of solely relying on recyclable practices. Utilizing sustainable processes is vital for creating medical packaging since recycled content is unsafe. A few factors that can be further looked into to create more sustainable methods are to make packaging more lightweight, use renewable energy, eliminate waste when possible, and recycle (Haub).
References
Amcor.com, https://www.amcor.com/media/news/amcor-healthcare-amsky-blister-system-wins-2022-sustainability-award.
“Business Wire.” Press Release Distribution, EDGAR Filing, XBRL, Regulatory Filings | Business Wire, https://www.businesswire.com/.
“Definition of Sustainable Packaging – SPC”. Sustainable Packaging Coalition, Aug. 2011, https://sustainablepackaging.org/wp-content/uploads/2017/09/Definition-of-Sustainable-Packaging.pdf.
“Different Types of Packaging Products [a Complete Guide].” Bizongo, https://www.bizongo.com/blog/different-types-of-packaging-products.
Haub, Catherine. “Sustainable Solutions for Medical Packaging.” Placon, 11 Aug. 2022, https://www.placon.com/resources/news/sustainable-packaging-solutions-for-medical-packaging/.
Larsen, Curtis L. “Effective Development of Terminally Sterilized Medical Device Packaging.” Medical Design Technology, vol. 10, no. 4, Apr. 2006, pp. 14–16. EBSCOhost, search.ebscohost.com/login.aspx?direct=true&db=asn&AN=20538025&site=ehost-live&scope=site.
“Packaging.” Wfhss Guidelines, https://wfhss-guidelines.com/packaging/.
Proclinical.com, https://www.proclinical.com/blogs/2022-9/the-top-10-medical-device-companies-in-the-world-in-2022.
“Reusable Critical Med Devices”. https://www.albertahealthservices.ca/assets/healthinfo/ipc/hi-ipc-sop-pkg-reusable-critical-med-devices-z0-res-topics-mdr.pdf
Richards, Dan. “Challenges and Strategies of Sustainable Packaging.” Skymark Packaging, Dan Richards Https://Www.skymark.co.uk/Wp-Content/Uploads/2021/07/Skymark-Logo-Col-300x138.Png, 16 Dec. 2022, https://www.skymark.co.uk/challenges-and-strategies-of-sustainable-packaging/#:~:text=One%20of%20the%20main%20challenges,than%20just%20the%20upfront%20costs.
Sharfman, Mark P., et al. “The Introduction of Postconsumer Recycled Material into Tyvek® Production, Marketing, and Organizational Challenges.” Journal of Industrial Ecology, vol. 5, no. 1, 2001, pp. 127–146., https://doi.org/10.1162/108819801753358544.
Sparrow, Norbert | Dec. “Sustainability in Medical Device Packaging? Yeah, It's a Thing.” Plasticstoday.com, 17 Dec. 2020, https://www.plasticstoday.com/medical/sustainability-medical-device-packaging-yeah-its-thing.
“Sterile Packaging.” Medical Device Manufacturer, https://www.spectrumplastics.com/components-technology/flexible-packaging-film/sterile-packaging/.
“Sustainability.” Mt. SAC, https://www.mtsac.edu/sustainability/.
“Sustainable Healthcare Packaging: Why Recyclability Is the Key.” Reusable Packaging News, 13 Dec. 2021, https://packagingrevolution.net/sustainable-healthcare-packaging/.
Terry, Beth, and Beth Terry. “Recycling Tyvek: Another Small Way to Deal with Plastic at Work (and Home) " My Plastic-Free Life.” My Plastic-Free Life, 7 Jan. 2018, https://myplasticfreelife.com/2007/10/recycling-tyvek-another-small-way-to/.