Products require very close attention when being shipped to avoid damages. Food and medicine require close supervision when being shipped due to sterilization standards and hazardous of potential poisoning and spoilage of foods. Atmospheric conditions cause severe ravages on packages in transit even with close monitoring and pre-shipment testing on materials. Standard packages are usually shipped in composite materials such as corrugated rigid paperboard or polyethylene bags. The most important concern for shipment materials is the likelihood of deterioration or dilution of distributed medical supplies and hazardous chemicals that could be wasted during the handling procedure during shipment.
Couriers that focus on temperature-sensitive packaging distribution require special shipment solutions such as climate-controlled trailers with specially equipped refrigeration systems that are insulated specifically for those items. Degassing valves are used to maintain freshness of materials, and to minimize potential problems such as wear and tear on products, time restraints, and exterior impacts. All elements are factored in to determine the most suitable and economically reasonable process for the transporting. Thermal engineers and packaging professionals are hired for testing materials that exceed satisfactory quality for heat transfer. If a product is shipped in a preferred physical state, it should remain as such for the entire duration of its shipment. This includes liquids that are frozen and vaccines that must be curated under certain temperatures. Depending on the product being shipped, materials of insulators in a package may vary by specification due to the molecular resistance of products. The goal is to maintain freshness and original physical condition of packages. Vaccines and syringes are closely monitored due to the fact they contain very sensitive bacteria and microorganisms that could be contaminated during international shipment. Outside factors such as temperature change and humidity are considered in manufacturer guidelines. Standard temperature for storage ranges between -20C and +70C. Similarly, the operating temperature ranges from -20C and +55C under ambient atmospheric conditions. Volume-per-dose requirement maximizes the availability for tertiary packaging.

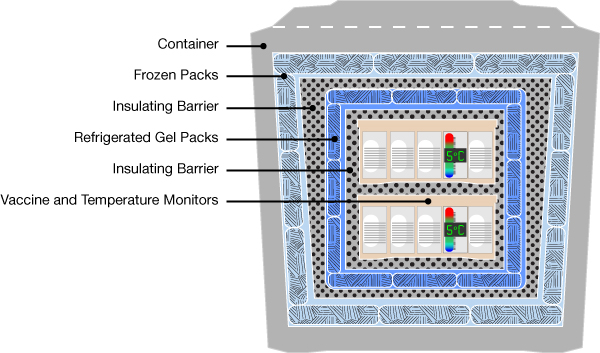
Problems beyond proper storage of vaccines have become prevalent in the past decade. Vaccines are becoming impotent due to the lack of proper handling and care once distributed from manufacturers. Most common factors of loss are corrosion, leakage, and mismanagement. Diseases and viruses that are usually curable have been deemed as incurable due to a lack of caution from handlers whom of which do not follow proper protocols. This situation is hurting the pharmaceutical industry by causing a financial loss in wasted immunizations. Proper training could reduce this cost. For instance, someone who oversees equipment inspections should always perform under the strict supervision of an experienced medical practitioner on duty when handling materials. Vials, or the containers that hold the content of vaccines should be properly disposed of during auditing procedures and should not appear in stock balances. Vaccines in “good” condition should be stored in stand-alone refrigerant units that are big enough to hold an estimated year’s quantity of a specific drug. External storage should be well ventilated with a calibrated digital logger. The digital logger stores data changes in atmospheric conditions so they can be monitored. A large change in temperature could be a perilous factor to the conservation of vaccines.
Transporting vaccines is a very challenging practice because of the risk of encountering wastage. They require extreme fragility and cannot be frozen with anything that interferes with their chemical composition. Usually, portable freezers are utilized but are direly required to keep the vaccines under a constant temperature throughout the entire span of their relocation.
Packaging of food is the most critical because it is essential for all individuals’ wellbeing. Manufactures in the food industry use nearly all the different packaging materials for the preservation of foods, to maintain freshness, and maximize shelf-life. Chemical exposure is one of the main causes of impurity. Exposures to external gases can alter the natural freshness of food and accelerate spoilage by breaking down the barrier that prevents pathogens that cause aging. Foil, glass, aluminum, plastic, and Styrofoam are the most commonly used packaging agents due to their ability to facilitate gases and block harming elements. They are easy to manipulate and are great gas conductors. Processed food requires special care because wastage could occur if not handled properly. The World Packaging Organization estimates more than 25% of wasted food comes from faulty packaging. Food packaging, as in other packaging, is separated into four major categories as listed based on varying similarities in characteristics: primary, secondary, tertiary, and unit load.
Primary packaging focuses primarily on precise and effective storage and distribution. Paper boxes and plastic containers are the basic storage compartments whose functionalities are simple, and usually meant for single-use only. They are also used for protecting stored items from outside factors that could alter freshness of food. Secondary, however, utilizes primary but adds a bit more complexity for grouping similar objects. Six pack rings from soda cans are examples of items that can be considered as secondary. Tertiary is used for distribution. The standard, corrugated cardboard box is usually the typical shipping method for efficient transportation. Unit load is a group of tertiary boxes that could easily be moved around in warehouses and lifted with proper handling equipment. One of the most essential, widespread materials used for food packaging is polyethylene (PE). PE is a thermoplastic that is very versatile and economically reasonable. It can withstand cold and somewhat extreme temperatures to preserve processed and organic foods. High and low-density PE are in simpler terms, used specifically for items that are compatible with certain molecular weights. Its strength is measured by crystallinity which determines yield strength and overall inelasticity. The temperature of a commodity can transform the physical state of PE if its polymer density is too high or too low.
Packaging has its complexities and requires a bit of science and lab testing to determine the best suitable solution for transportation. With proper care and expertise, the amount of wastage of goods can be greatly reduced.
References:
Centers for Disease Control. Vaccine Storage and Handling Toolkit. http://www.cdc.gov/vaccines/recs/storage/toolkit/ storage-handling-toolkit.pdf. web. Marsh, Kenneth. “Food Packaging—Roles, Materials, and Environmental Issues.” Institute of Food Technologists. 2007. Print.
Shin, Joongmin and Selke, Susan, E.M. “Food Packaging.” Food Processing: Principles and Applications, Second Edition. John Wiley and Sons. 2014. Print.
Singh, S. P., Burgess, G., and Singh, J. (2008), “Performance Comparison of Thermal Insulated Packaging Boxes, Bags and Refrigerants for Single-parcel Shipments.” Packaging Technology and Science, 21: 25-35.
World Health Organization. “Monitoring Vaccine Waste at Country Level”. Access to Technologies Team. November 2003. Print.
“Guidelines on the International Packaging and Shipping of Vaccines.” Access to Technologies. World Health Organization. Dec. 2005. Print.